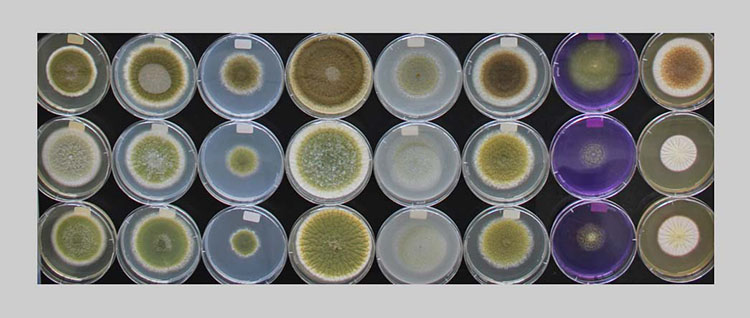
At the Zurich University of Applied Sciences in Wädenswil, bakery relevant moulds were isolated, which can be used to test antifungal sourdough cultures.
Bread and other bakery products with an aw-value between 0.93 and 0.96 and a pH-value between 5 and 6 provide optimal growing conditions for moulds. As a result, spoilage due to moulds is a major contributor to the reduced shelf life of bread products (Santos et al., 2017).
If bread and bakery products are contaminated by moulds, it usually occurs after they have been baked in the oven, since mould spores that come with flour are killed by the high baking temperatures. Rather, this usually takes place during the transport, cooling, storage, cutting and packaging of the products. The spores are most commonly transmitted to the bread products by air, devices and machines, packaging materials and bakery employees (Müller, 1997; Schünemann et al., 2016).
Biocontrol for mould prevention
To extend the shelf life of bread and baked goods preservatives are often used. Both, in Switzerland and in the EU, up to 3000 mg/kg propionate and up to 2000 mg/kg sorbate are allowed in packaged sliced bread and rye bread. The use of acetic acid and acetates, lactic acid and lactates is allowed according to good manufacturing practice (quantum satis) in breads made from wheat flour, water and yeast or sourdough and salt (Regulation (EC) No 1333/2008; ZuV, 2013).
However, using chemical preservatives to extend the shelf life of products is being increasingly criticized, and there is also a trend among consumers to seek out natural food products. A possible alternative to prevent mould growth is biocontrol, which has become increasingly interesting in recent years. With biocontrol, microorganisms or their metabolites are specifically used to suppress spoilage or pathogens. In the baked goods sector, sourdoughs are suitable for this purpose, since they can be fermented using antifungal cultures, especially lactic acid bacteria, and then added to the baked goods (Megan et al., 2012; Axel et al., 2017).
Internationally, delayed mould growth on baked goods by use of antifungal sourdough has been described by several research groups (Gerez et al., 2009; Ryan et al., 2011; Garofalo et al., 2012; Le Lay et al., 2016; Luz et al., 2019). In addition to the fact that this approach is sustainable and inexpensive, it complies with clean labeling principles. Furthermore, sourdoughs generally create value for the consumer through the improved sensory and nutritional properties that it adds (Rollán et al., 2010; Zannini et al., 2012).
However, this begs the question, which moulds should be used for the development of such antifungal sourdoughs? The answer is simple: the moulds that are product relevant and that should be prevented in practice should be used.
In a study conducted by the Zurich University of Applied Sciences (ZHAW), airborne mould contamination was evaluated in Swiss bakeries as a possible source of bread contamination. In addition, mould isolates from the air and from bread loaves at industrial bakeries were isolated and identified. The overall goal was to document bakery relevant mould types, in order to develop future strategies for targeted prevention, such as biocontrol with antifungal sourdoughs
Air and Bread analysis
In a first step, to determine the extent to which moulds in the ambient air is able to contaminate breads, the air was tested in five industrial Swiss bakeries at three different sampling locations (A, dough preparation; B, before or in the bread proofer; C, after the baking process or before packaging) using passive and active methods. Active air samples were taken at the above-mentioned locations using a MAS-100 NT air sampler (MBV, Stäfa). In each case, both 100 and 500 litres of air were actively drawn in. For the passive method, petri dishes containing suitable nutrient media were left open on a flat surface at the same sampling locations for 60 minutes. After 5 days of incubation at 25 °C, the colony-forming units (CFU) on the petri dishes were enumerated and the moulds were documented.
Beside the air analysis, between 5 and 20 loaves of bread from each bakery, which had been baked and packaged at the same time as the air samples were taken, were randomly selected and stored for 4 to 7 days at room temperature. With one exception, no preservatives were used in the selected loaves. All breads were made with wheat flour, sometimes with a small amount of rye flour, and in one case with corn flour. The aw-value of the examined breads varied between 0.89 and 0.94 and the pH-value was between 5.52 and 5.67.
Subsequently, all moulds that had been isolated from the air at sampling location C (100 l) in two of the bakeries and a selection of moulds that had developed on the loaves at three of the bakeries during storage were isolated on malt extract agar [20 g/l malt extract broth (Biolife, 4016602), 15 g/l Agar Bacteriological (Biolife, 4110302)]. Pure mould isolates were integrated into the strain collection maintained by the Food Biotechnology Research Group and identified by sequencing the ITS region and the β-tubulin gene. Further details on the procedure can be found on the website titled baking + biscuit international at www.bakingbiscuit.com/additionl-information.html
Mould contamination in the air at industrial Swiss bakeries
The data from the active air samples (Figure 2) showed that the mould concentration in each bakery was lower at sampling location A (dough preparation) and fluctuated less than at sampling location B (before/in the bread proofer) and C (after the baking process or before packaging). Air samples from location A could only be taken in three of the five bakeries. Depending on whether Dichloran 18% Glycerol (DG18) Agar (Oxoid, CM0729) or Dichloran Rose-Bengal Chloramphenicol (DRBC) (ISO) Agar (Oxoid, CM1148) was used, mould concentrations ranged from 70 to 170 CFU/m³ and from 10 to 470 CFU/m³, respectively. Air samples could only be taken at four of the five bakeries in sampling location B, with the median mould concentration varying between 230 and 245 CFU/m3 when using DG18 or DRBC, respectively. The individual values fluctuated between 30 and 1240 CFU/m3 using DG18, and 0 and 1010 CFU/m3 using DRBC. At sampling location C, the mould concentrations in the air were between 10 and 1390 CFU/m3, depending on the bakery. The median value varied between 270 and 243 CFU/m3, depending on the nutrient medium. In one of the bakeries, the mould concentration levels at sampling location C could not be determined. The highest values shown in Figure 2 for sampling location B (1240 CFU/m3) and C (1390 CFU/m3) were estimated, as the plates showed very strong growth and enumeration was affected.
To date, only a few studies have investigated mould contamination in bakeries or other food-production facilities. In comparison to the data collected in the present study, an analysis of German bakeries showed that the air in bread storage rooms can have an average spore concentration of 85 to 5000 mould spores/m3. In some production areas, up to 90,000 CFU/m3 has even been measured (Legan, 1993). Since there are no guidelines outlining acceptable mould concentration levels in the air of food production facilities in Switzerland or the EU, the values cannot be interpreted in a positive or negative manner. Beyond that, mould concentrations in the air can vary widely over the course of a day, and depend on a variety of factors, such as weather conditions, ventilation, and the number of people and activities that take place in an area (Legan, 1993).
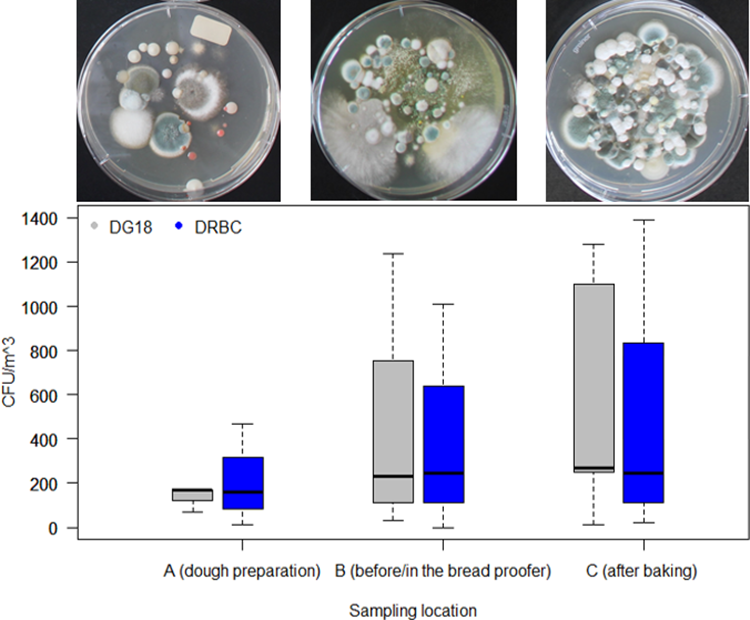
Boxplot of bakery mould concentration in the air at three sampling locations: A (dough preparation), B (before or in the bread proofer) and C (after the baking process or before packaging) determined by active (100 l) air sampling using DG18 (grey) and DRBC (blue). The median is indicated by the black line in each box
In the passive air samplings (Figure 3), the average number of mould spores that were deposited per hour in sampling locations A and B varied between 1 and 3 CFU/h in all of the bakeries examined. As already observed in the active samplings, sampling location C (after the baking process or before packaging) showed higher values. The median concentration was between 11 and 12 CFU/h, depending on whether DG18 or DRBC was used. This indicates that bread loaves in the packaging area are contaminated at a rate of between approx. 0.17 and 0.19 spores/cm2/h. In comparison, Spicher (1980) found that loaves in German bakeries were contaminated at a rate of between 1 and 4 spores/cm2/h.
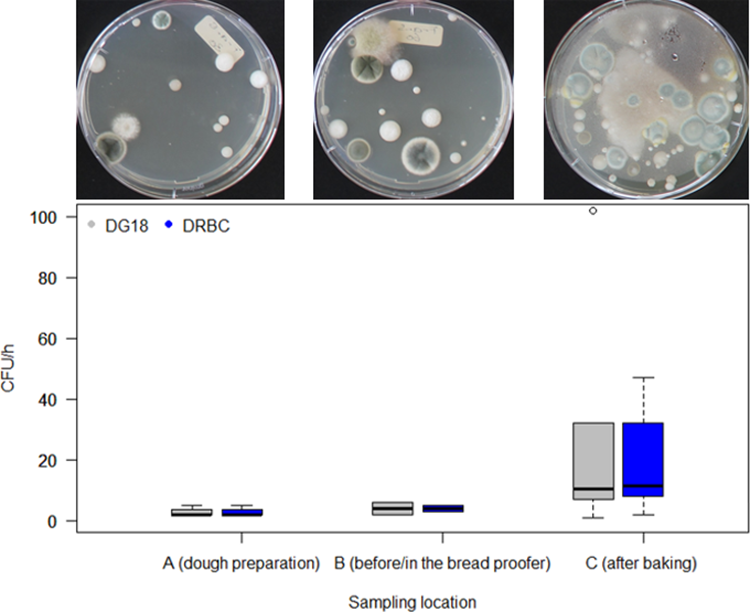
Boxplot of bakery mould concentration in the air at three sampling locations: A (dough preparation), B (before or in the bread proofer) and C (after the baking process or before packaging) determined by passive air sampling over 1 hour using DG18 and DRBC. The median is indicated by the black line in each box
Collection of moulds relevant to bread
A total of 299 moulds were isolated through the active and passive air tests at sampling location C, and an additional 113 moulds were taken from the loaves. Following macroscopic assessment, the isolated moulds were divided into 51 groups. Of the 412 moulds, 127 could not be assigned to any group. A representative sample from each of the groups, as well as the samples which could not be assigned to any group (195 samples in total), were identified by sequencing of the ITS region and the β-tubulin gene. Of the 412 isolated moulds, 12 bread isolates and 95 air isolates could not be identified.

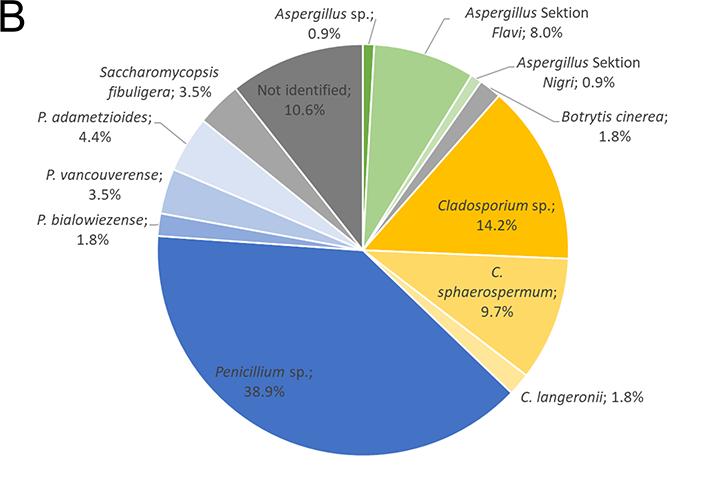
Identified isolates from the air (A) and bread (B). The isolates were identified by gene sequencing (IST region and β-tubulin gene)
Concerning the airborne moulds (Figure 4 A), 33.1% were found to be from the Penicillium genus, 18.7% were from the Aspergillus genus, 10.7% were from the Cladosporium genus, and 5.7% belonged to various other genera like Alternaria spp. (1.3%), Mucor spp. (0.7%), Rhizopus microsporus (0.3%), Lichtheimia spp. (0.3%), Paecilomyces variotii (0.3%) and Trichoderma spp. (1.3%). 31.8% of the isolates could not be identified. Of the identified moulds, 48.5% were Penicillium, 27.5% were Aspergillus, 15.7% were Cladosporium, and 8.3% other genera. Similar to this study, the prevalence of the genera Penicillium, Aspergillus and Cladosporium in the air at bakeries has also been observed in studies carried out by other research groups in different countries (Garcia et al., 2019; Cornea et al., 2011; Singh & Singh, 1994). These findings confirm the relevance of these genera to the bakery industry.
Of the moulds isolated from the bread loaves (Figure 4 B), 48.7% were found to be of the Penicillium genus, 25.7% belonged to the Cladosporium genus, 9.7% were from the Aspergillus genus, and 1.8% belonged to other genera. 3.5% of the isolates were identified as the yeast Saccharomycopsis fibuligera and 10.6% of isolates could not be identified.
In a study in Great Britain, the presence of mould on bread loaves in 46 bakeries over the course of a year was determined. Similar to the present study, Penicillium spp. and Aspergillus spp. were frequently observed. Penicillium spp. was found on nearly every loaf and Aspergillus spp. and Cladosporium spp. were found on half of the loaves examined (Legan & Voysey, 1991).
About 50% of the Penicillium sp. isolated from the bread loaves is probably the species P. crustosum, the Aspergillus in the Flavi section are probably the specie A. flavus and the Aspergillus in the Nigri section are probably A. niger, since these species were the most common hits in the BLAST searches. P. crustosum is very common indoors and has been isolated in chocolate factories in Belgium (De Clercq et al., 2015). A. flavus and A. niger were also isolated from bread in an industrial bakery in a Danish study and accounted for 3.3% (156:4687) and 2.8% (129:4687) of the isolated moulds over 4 years (Lund et al., 1996). According to the literature, P. crustosum and A. flavus, as well as A. niger are typical causers of bread spoilage (Reiss, 1973). One of the properties of P. crustosum is its ability to form Roquefortin C, a neurotoxic substance (Kokkonen et al., 2005). In addition, many strains of the A. flavus species are able to produce the mycotoxin aflatoxin (Varga et al., 2011) and A. niger can generate ochratoxin A (Frisvad et al., 2011).
Conclusion
The results of the current study illustrate the current airborne mould concentration levels in five industrial Swiss bakeries and provide an overview of the dominant mould flora both in the air and on bread. The prevalence of Penicillium, Aspergillus (especially the Flavi section) and Cladosporium observed both in the air and on the bread loaves indicates that the mould in the air at bakeries is a possible source of bread contamination. The moulds identified over the course of this study are representative of the moulds that cause bread spoilage and are suitable for use in future screening and challenge tests for the development of antifungal sourdoughs in a biocontrol strategy.
Authors
Susette Freimüller Leischtfelda,
Susanne Miescher Schwenningera
Food Biotechnology Research Group, Institute for Food and Beverage Innovation, Zurich University of Applied Sciences (ZHAW), Wädenswil, CH


